This fluorescent technique can precisely measure minuscule distances
Steffen J. Sahl / Max Planck Institute for Multidisciplinary Sciences
The tiniest “ruler” ever is so precise that it can measure the width of a single atom within a protein.
Proteins and other large molecules, or macromolecules, sometimes fold into the wrong shape, and this can affect the way they function. Some structural changes even play a role in conditions like Alzheimer’s disease. To understand this process, it is important to determine the exact distance between atoms – and clusters of atoms – within these macromolecules, says Steffen Sahl at the Max Planck Institute for Multidisciplinary Sciences in Germany.
Advertisement
“We wanted to go from a microscope that maps positions of macromolecules relative to each other, to taking this bold step of going within the macromolecule,” he says.
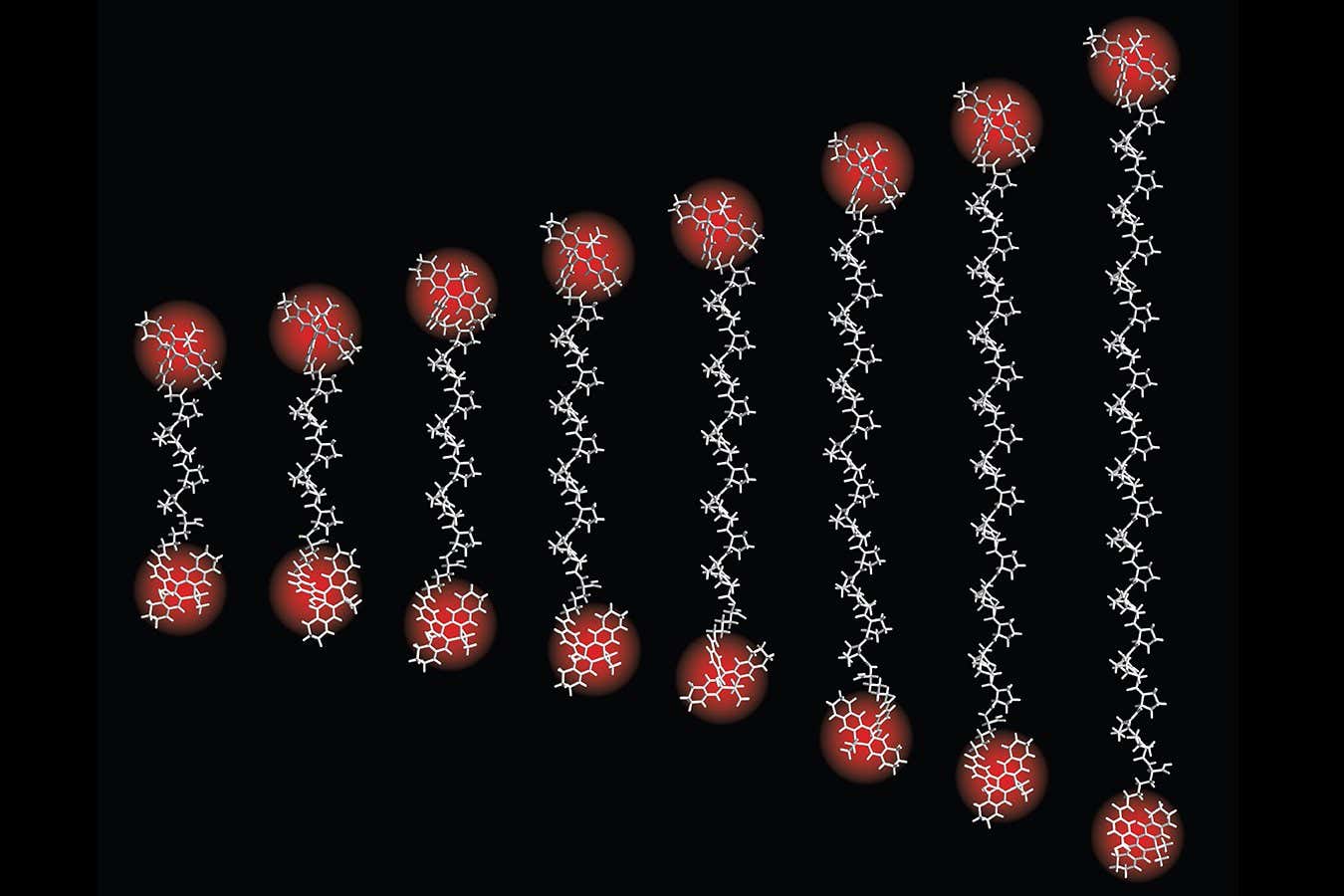
To construct their intramolecular “ruler”, Sahl and his colleagues used fluorescence, or the fact that some molecules glow when illuminated. They attached two fluorescent molecules to two different points on a larger protein molecule and then used a laser beam to illuminate them. Based on the light the glowing molecules released, the researchers could measure the distance between them.
They used this method to measure distances between the molecules of several well-understood proteins. The smallest of those distances was just 0.1 nanometres – the width of a typical atom. The fluorescent ruler also gave accurate measurements up to about 12 nanometres, meaning it had a broader measuring range than can be achieved with many traditional methods.
In one example, the researchers looked at two different forms of the same protein and found that they could distinguish between them because the same two points were 1 nanometre apart for one shape and 4 nanometres apart for the other. In another experiment, they measured tiny distances in a human bone cancer cell.
Sahl says the team achieved this precision by taking advantage of several recent technological advances, like better microscopes and fluorescent molecules that don’t flicker and don’t produce a glow that could be confused with some other effect.
“I don’t know how they got their microscopes so stable. The new technique is definitely a technical advance,” says Jonas Ries at the University of Vienna in Austria. But future studies will have to determine for which exact molecules it will prove most useful as a source of information for biologists, he says.
“While it boasts impressive precision, the new method may not necessarily achieve the same level of detail, or resolution, when applied to more complex biological systems,” says Kirti Prakash at The Royal Marsden NHS Foundation Trust and Institute of Cancer Research in the UK. Additionally, he says that several other new techniques are already becoming competitive in terms of measuring smaller and smaller distances.
Sahl says his team will now work on two tracks: refining the method further and expanding their ideas about which macromolecules they can now peer inside.
Topics: